- Home
- Sustainability
- Social and Environmental Report (Download)
- Aim to provide products to be chosen for being “Reliable” - Social Responsibility 2014
Aim to provide products to be chosen for being “Reliable”Social Responsibility 2014
The Nisshin Seifun Group believes that our top priority is to provide reliable and safe foods to our consumers. We are committed to secure safety of various products including wheat flour, cake mix, pasta, frozen foods and health foods to ensure that consumers can truly enjoy their foods.
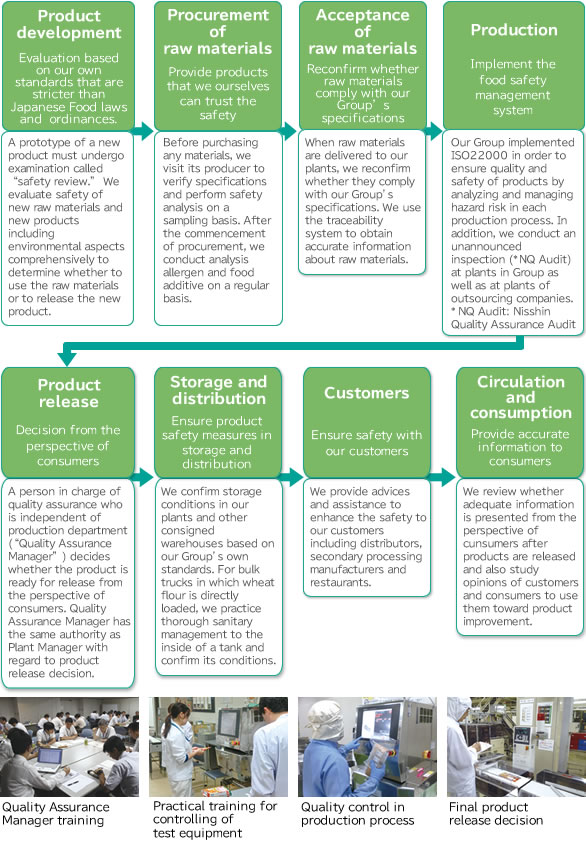
Consistent quality assurance from development and procurement through distribution
To secure reliability of food, our Group ensures the safety, including food defense, at every step of the entire process from product development, procurement of raw materials through distribution, and places the highest priority to quality assurance from the perspective of consumers.
Various efforts to ensure food safety
Obtain certification of food safety management system
The Nisshin Seifun Group works on implementing ISO22000*, the global standard for the food safety management system.
In February 2014, Oriental Yeast Co., Ltd. and Pany Delica Co., Ltd. newly acquired certification.
*ISO22000: A new food safety management system that incorporates HACCP’s hazard analysis method for food safety that are recognized globally in the food sanitary management.
| Year | Scope |
|---|---|
| November 2005 | Nisshin Flour Milling Inc. (headquarter and 11 domestic plants) It is the first case among the food manufacturer in Japan. |
| 2007 | Nisshin Foods Inc., Ma・Ma-Macaroni Co., Ltd. and Initio Foods Inc. |
| August 2008 | Shin Nisshin Seifun Foods (Qingdao) Co., Ltd. It is the first certification among our overseas affiliate of the Nisshin Seifun Group. |
| February 2014 | Oriental Yeast Co., Ltd. and Pany Delica Co., Ltd. |
Audit under AIB Consolidated Standard for Food Safety
AIB Consolidated Standard for Food Safety is a food safety guidelines and inspection system developed by American Institute of Baking (“AIB”). The inspection ensures that an entity has a system to supply safe products in processes from acceptance of raw materials through product release, focusing on production sites. The Nisshin Seifun Group adopted this standard in 2001, and currently, a number of plants utilize it to maintain and improve the product safety management level.
Quality assurance training to raise awareness of our employees
To ensure food safety, it is essential to make our employees build awareness of it. The Nisshin Seifun Group provides quality assurance trainings every year to all officers and employees who are engaged in research and development, production and marketing activities in our Group companies. In order to gain a better understanding of quality assurance and ensure to put it into practice in daily operations, the trainings offer broader range of knowledge on food safety, including the general sanitary management method, our Group’s guidelines and rules, and trends of the industry and relevant legislation.
Ensure traceability
Our Group endeavors to ensure traceability (production history management) that records and manages various data about raw materials, production, storage and distribution. It enables us to improve “reliability of information” through accurate and detailed information management, and also it ensures “food safety,” in case a serious accident, should happen by prompt cause investigation and quick product recall.
For example, in premix plants of the Nisshin Seifun Group, a bar code label containing information about raw materials is attached to all raw materials for identification purposes. By reading bar code information when the raw materials are used, we can manage the history of all raw materials used for the products. This system enables us to trace back from products to raw materials and trace forward from raw materials to products accurately and promptly.

Ueda Plant received the certification of “GMP on Dietary Supplement and Raw Materials* ”
The Ueda Plant of Nisshin Pharma Inc. acquired the certification of “GMP on Dietary Supplement and Raw Materials” from Japan Health and Nutrition Food Association. “GMP on Dietary Supplement and Raw Materials” is a guideline to provide standards for production and management of entire processes from acceptance of raw materials through packing and product release, and Japan Health and Nutrition Food Association examines manufacturing facilities whether production and quality management meet the guideline. Since March 2012, the Ministry of Health, Labor and Welfare started to recommend consumers to purchase dietary supplements manufactured in GMP-certified plants.
*GMP (Good Manufacturing Practice) is guidelines or standards for production and quality management